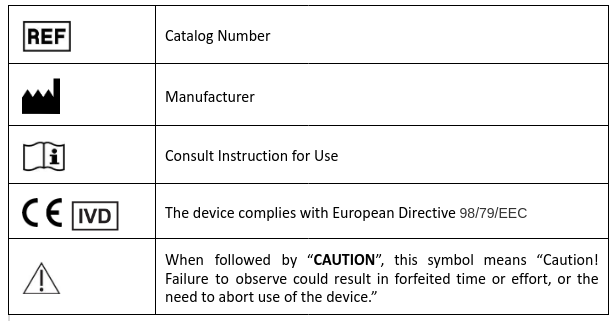
Explanation of symbols
Introduction
This document provides general information and procedure to use VarSome Clinical, available by using the following URL:
https://clinical.varsome.com/
⚠ CAUTION These Instructions for Use (IFU) contain important information for the safe use of this product. Please read the entire IFU before using our product.
Manufacturer:
Saphetor SA
EPFL Innovation Park – C 1015 Lausanne
+41 21 552 09 77
www.saphetor.com / www.varsome.com
Authorized Representative
Saphetor SA Greek Branch
Leocharous 3 - Athens 105 60
Greece
Result Validation
We can provide detailed validation results for known individuals whose cells/DNA can be ordered by post here.
The samples should be run through normal laboratory procedures. Then send us the FASTQ files, and we will validate the variants against the Genome in a Bottle (GIAB) dataset giving detailed specificity and sensitivity.
Regulatory Information
According to the Annex III of the European Directive 98/79/EEC, VarSome Clinical is considered as an In-Vitro Medical device.
The use of VarSome Clinical is subject to the terms and conditions provided on our website. Please consult them.
